The 2004 NIOSH ALERT: Preventing Occupational Exposures to Antineoplastic and Other Hazardous Drugs in Health Care Settings revealed devastating news to 5.5 million healthcare clinicians exposed to hazardous drugs (HD) at work. The evidence was clear. Workplace exposure to HD is associated with negative health effects including cancer, skin rashes, and adverse reproductive outcomes (including infertility, spontaneous abortions, and congenital malformations). While alarming, this article also empowered clinicians to take evidenced-based action to safeguard themselves from exposure, recommending a safety program including the use of Closed System Transfer Devices (CSTD) for hazardous drug protection. Nearly twenty years later, this month marks the next milestone in protecting healthcare workers, when USP General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings finally becomes a nationally enforceable standard. USP <800> requires that a CSTD is used when a HD is administered and recommends them for HD preparation. Reading the NIOSH report in 2004 marked the beginning of an inspiring journey for Todd Korogi, a mechanical engineer from northeast Ohio. The warnings in the NIOSH report inspired a sketch of a CSTD at Todd’s kitchen table and prompted a Thanksgiving break design challenge that would develop into a CSTD that has simplified hazardous drug protection across the world.
The NIOSH alert presented a problem and sparked innovation. Todd had co-founded Gilero, a medical device contract development and manufacturing service company, and presented his partner Ted Mosler with a design challenge before Thanksgiving break. The challenge was both simple and complex. Develop a product to protect healthcare workers (HCW) from the harms of HD by addressing risks during the administration process and focusing on exposure points at the syringe or IV administration set.
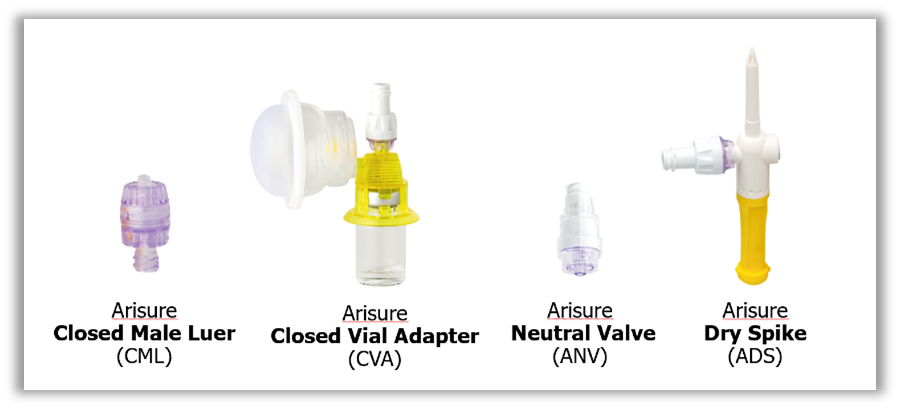 This design challenge was the genesis of the Arisure® Closed Male Luer and the Arisure® Closed System Transfer Device (CSTD), offering protection to millions of clinicians with a simple, yet innovative approach to hazardous drug protection. The journey here was paved with determination, alignment with critical experts, and focused dedication to a simple mission: to be the best in the world at moving medicine safely.
This design challenge was the genesis of the Arisure® Closed Male Luer and the Arisure® Closed System Transfer Device (CSTD), offering protection to millions of clinicians with a simple, yet innovative approach to hazardous drug protection. The journey here was paved with determination, alignment with critical experts, and focused dedication to a simple mission: to be the best in the world at moving medicine safely.
They began by consulting experts in HD exposure, preparation, and administration across the globe. They consulted thought leader Martha Polovich, a co-author of the Oncology Nurses Society Handbook for Hazardous Drug Safety at the time. She generously shared her knowledge and expertise on the primary exposure points in the process of preparing and administering HD drugs in real practice settings. Through engaging with practicing clinicians and experts, it was clear that the product needed to be extremely effective at being dry, very intuitive to use, minimize disruptions to workflow, and function without the use of needles, thereby eliminating sharps injury risk. A few years later, Todd’s vision became even more clear when speaking with a group of HCWs in Brazil about the risks of HD exposure where he met a woman who was overwhelmed with emotion. As her story was translated from Portuguese, Todd learned of her struggles with infertility after caring for cancer patients, and his dedication and determination for solving the problem became unrelenting. In 2008 Todd founded Yukon Medical, a nimble and diverse team of innovators and critical thinkers, with a passion to make a difference in the lives of patients and caregivers. Today the Arisure® Closed System Transfer Device (CSTD) is simplifying hazardous drug protection for healthcare facilities across the world.
Arisure® CSTD: Designed to Simplify Hazardous Drug Protection
The Arisure® CSTD was designed to simplify HD protection using a needle-free, mechanically and microbiologically closed system to enable airtight and leakproof transfer of hazardous medications. The team at Yukon Medical followed the FDA’s lead after they established the ONB product code for “closed antineoplastic and hazardous drug reconstitution and transfer systems” and established FDA clearance in this newer category of devices. The CDC (NIOSH), also understanding the unique risks posed by HD have originated and updated several draft protocols for assessing CSTDs. The expectations for CSTD have evolved over the last decade but Yukon Medical has remained dedicated to consistently meeting or exceeding hazardous drug safety requirements including product performance testing, as outlined by both regulatory agencies, while also consistently addressing critical end-users needs including reliability, ease-of-use and workflow impact.
With easy-to-use, intuitive luer lock connectivity and advances in CSTD compatibility, the Arisure® CSTD is expanding protection for nursing and allowing pharmacy to adopt a passive hazardous drug protection program. The Arisure Closed Male Luer is compatible with *BD™ SmartSite™ and **Baxter ONE-LINK & CLEARLINK connectors, present on BD Alaris™, Baxter™, and Zyno™ infusion pump sets, and widely used across the U.S. Together with these compatible connectors, the Arisure® CML achieves a dry, airtight and leakproof HD infusion. In a facility using IV sets that contain these compatible valves, a pharmacy can dispense the HD with the Arisure® CML on the end of the set or syringe, and the nurse can then connect the Arisure® CML directly to the compatible connector already on the patient to administer the infusion. This expanded compatibility allows the USP <800> CSTD requirement to be achieved passively, with no added action or interruption to nursing workflow. Reducing the number of components required to deliver a HD infusion may also improve cost, storage logistics, and simplify compliance for a healthcare facility. With the Arisure® CML, hazardous drug protection can be delivered passively without interrupting patient care. In facilities that do not use these infusion pumps or compatible needle-free connectors, the Arisure® Neutral Valve can be included in the HD safety program to enable a dry, closed, leakproof infusion.
Two major obstacles on the path to providing access to the Arisure® advantage to U.S. clinicians were finding the right specialty distribution partner and convincing HCWs to evaluate the simple and easy-to-use product when the alternative CSTD systems they were using were overly complex, often requiring more than a dozen parts or pieces to complete one HD infusion. At the end of 2021 Yukon partnered with Progressive Medical Inc. (PMI) to bring the Arisure® CSTD to U.S. Clinicians. A key component of this partnership includes access to a nationwide, clinically trained, specialized sales force supported by licensed clinicians and dedicated sales leadership. This clinical, focused approach provides a highly educated sales force and peer-to-peer clinician partnerships that deliver effective product integration and support customized to the needs and infrastructure of the healthcare facility. The passive safety and workflow advantage of Arisure® combined with customized and comprehensive integration and support by clinically trained experts has propelled Arisure®, Yukon® and Progressive Medical, Inc. into a HD protection powerhouse. Today Todd continues working directly with healthcare clinicians on the front lines caring for patients along with the PMI team, integrating the dependable Arisure® system seamlessly into practice.
As more healthcare facilities take advantage of the passive safety advantage of the Arisure® CSTD and adopt the CSTD to ensure compliance with USP<800> regulations, Arisure® is well-positioned to protect every clinician with the capacity, supply stability and reliability needed in today’s environment. Yukon Medical is a division of Industrie Borla (Borla) based in Torino, Italy. Borla is one of the world’s largest IV set component manufacturers supplying billions of components worldwide every year for over 50 years. Borla has built their business to be able to supply in high volume globally without disruption and Arisure® customers can integrate the solution confidently for continuous, quality hazardous drug protection. An alert to the dangers and consequences of HD exposure inspired a humble engineer to think big and successful partnerships have continued to pave the path forward for Arisure®, making it accessible to every exposed clinician and simplifying HD preparation and administration safety long before USP<800> required it.

*SmartSite is a trademark of Becton, Dickinson and Company, its subsidiaries or affiliates. PMI and Yukon Medical Inc. are not affiliated with Becton, Dickinson and Company.
**ONE-LINK AND CLEARLINK are trademarks of Baxter International Inc, its subsidiaries or affiliates. PMI and Yukon Medical Inc. are not affiliated with Baxter International Inc.

