PMI offers a range of Infusion Products designed to provide Value and Clinical Excellence.
Experts in the area of Infusion Therapy and IV Specialties, our clinically trained sales staff is supported by in-house clinical professionals. PMI’s synergistic product portfolio reduces healthcare cost by maximizing clinical efficiency and improving safety for patients, employees, and providers.
Yukon Medical
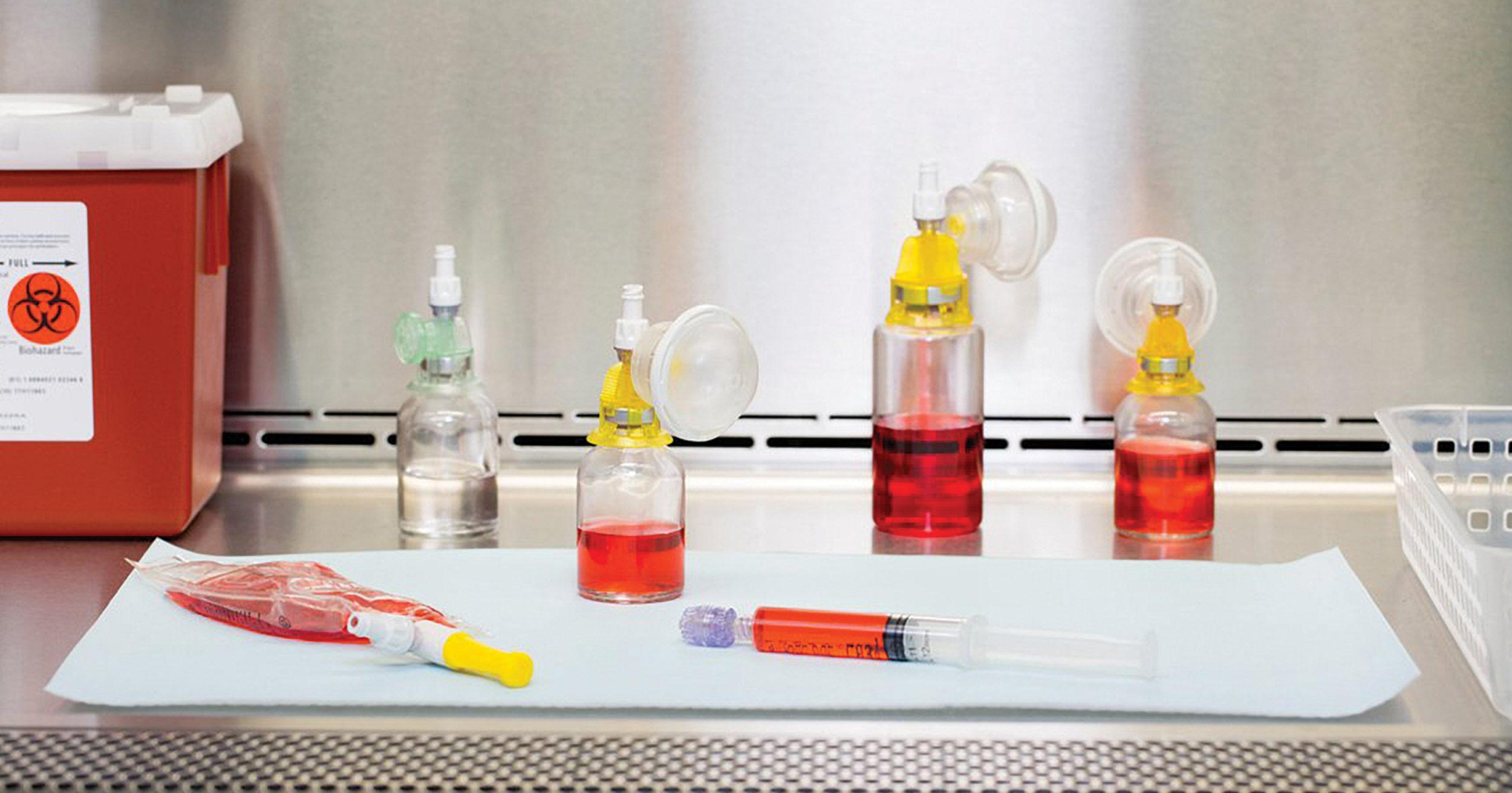
The Arisure® Closed System Transfer Device offers a novel, needle-free, dual membrane, airtight and leakproof technology for the safe, simple and secure preparation & administration of hazardous drugs. Arisure® is mechanically and microbiologically closed and can easily integrate into current practice to help ensure compliance. Compact design for ease-of-use, with fewer parts, pieces, and steps. The intuitive luer lock dry connections are easy to use for both pharmacy and nursing.
SMARTeZ®
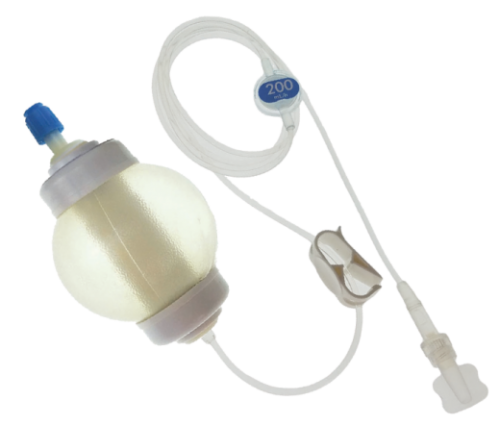
The safe, accurate, simple, solution for short duration, long duration, and home infusion therapy. SMARTeZ® disposable elastomeric pumps are intended for intermittent and continuous antibiotic infusion, chemotherapy, pain management and general infusion therapies.
Asset Medical

Asset Medical is a specialized company focused on infection control and safety of healthcare professionals. Our infusion therapy options, FlowArt® and NeutrArt®, are designed to aid in protecting the patient and nursing staff from exposures while improving quality of care.
PMI INFUSELINE®

PMI offers a variety of INFUSELINE® Products, ranging from flow regulator IV sets to pharmacy pump sets. Our product offerings are designed for patient-focused solutions and operational efficiency enhancement in IV infusion and pharmacy settings. Progressive Medical, Inc. is committed to improving clinical outcomes through our INFUSELINE® product offerings.
West Pharmaceuticals
![]()
The West Vial2Bag Advanced® Admixture Devices enable reconstitution and transfer of a drug between a vial and an IV bag using an integrated adapter prior to administration to the patient.
Prescription Use Only.
The Vial2Bag Advanced® admixture devices are 510(k) cleared by the United States Food and Drug Administration (FDA). The use of the Vial2Bag Advanced® admixture devices should not be interpreted as modifying, extending, or superseding a drug manufacturer’s labeling recommendations for storage and expiration dating, unless otherwise limited by USP <797> compounding standards. Refer to drug manufacturer’s labeling and use instructions for recommendations, USP <797>, and applicable institution policy for shelf life and sterility information of reconstituted product and admixture device compatibility. The Vial2Bag Advanced® admixture devices are not compatible for use with all drug products. Do not use the Vial2Bag Advanced® admixture devices with lipids. Failure to follow the instructions provided may result in inadequate medication reconstitution, dilution, and/or transfer, possibly leading to overdose or underdose and/or delay in therapy. Products shown are for INFORMATION purposes only and may not be approved for marketing in specific regions. Important product and safety information and warnings at: https://www.westpharma.com/products/vial-adapter-systems/vial2bag-advanced-admixture-adapter-drug-transfer-system.
Contact a Customer Service Specialist for more information
-or-
Complete the form below to request product information from your partners at PMI.
