 Decades of research reveal the devastating consequences of exposure to hazardous drugs (HD) for healthcare workers (HCW) preparing and administering them to patients. For over forty years, leading clinical organizations including USP, ASHP, ONS, and NIOSH have produced influential guidelines warning of the risks of HD exposure and providing evidence-based recommendations to protect our caregivers (Figure 1). A review of trends in adoption rates of Closed System Transfer Devices (CSTD) over the past twelve years indicates that far too many HCWs administering these drugs remain unprotected (Figure 2). In November 2023, the updated USP General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings finally becomes a nationally enforceable standard. Legally enforceable hazardous drug safety standards, along with advances in CSTD compatibility that address device complexities, nursing workflow and cost barriers, could make this the year every clinician is offered protection from the harms of HD.
Decades of research reveal the devastating consequences of exposure to hazardous drugs (HD) for healthcare workers (HCW) preparing and administering them to patients. For over forty years, leading clinical organizations including USP, ASHP, ONS, and NIOSH have produced influential guidelines warning of the risks of HD exposure and providing evidence-based recommendations to protect our caregivers (Figure 1). A review of trends in adoption rates of Closed System Transfer Devices (CSTD) over the past twelve years indicates that far too many HCWs administering these drugs remain unprotected (Figure 2). In November 2023, the updated USP General Chapter <800> Hazardous Drugs – Handling in Healthcare Settings finally becomes a nationally enforceable standard. Legally enforceable hazardous drug safety standards, along with advances in CSTD compatibility that address device complexities, nursing workflow and cost barriers, could make this the year every clinician is offered protection from the harms of HD.
Figure 1. TIMELINE OF HAZARDOUS DRUG SAFETY GUIDANCE
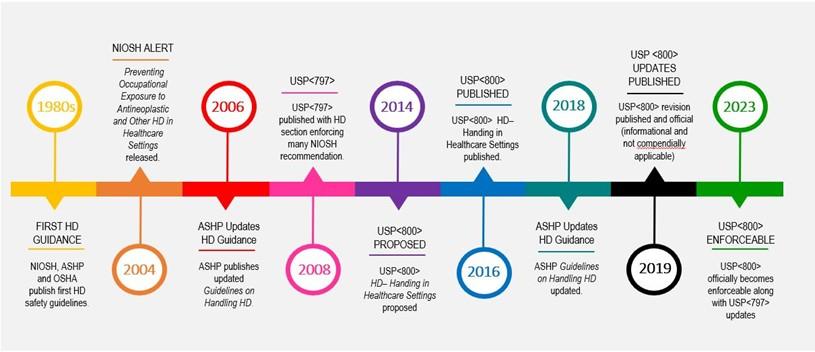
NIOSH: National Institute of Occupational Safety and Health. ASHP: American Society of Health-System Pharmacists.
OSHA: Occupational Safety and Health Administration. HD: Hazardous drug. USP: United States Pharmacopeia.
One category of hazardous drug safety required in USP <800>, and already recommended in many hazardous drug safety guidelines, is the use of Closed System Transfer Devices (CSTD). These medical devices are designed to protect HCWs and patients from exposure to HD. USP <800> requires CSTDs are used for HD administration and recommends that they are used in HD preparation. Pharmacy Purchasing & Products publishes results of an annual State of Pharmacy Compounding survey1. Compilation of their data on CSTD adoption rates for pharmacy and nursing beginning in 2010 indicates that we are not protecting the healthcare workers that administer HDs with the same rigor that we are protecting those preparing them (Figure 2).
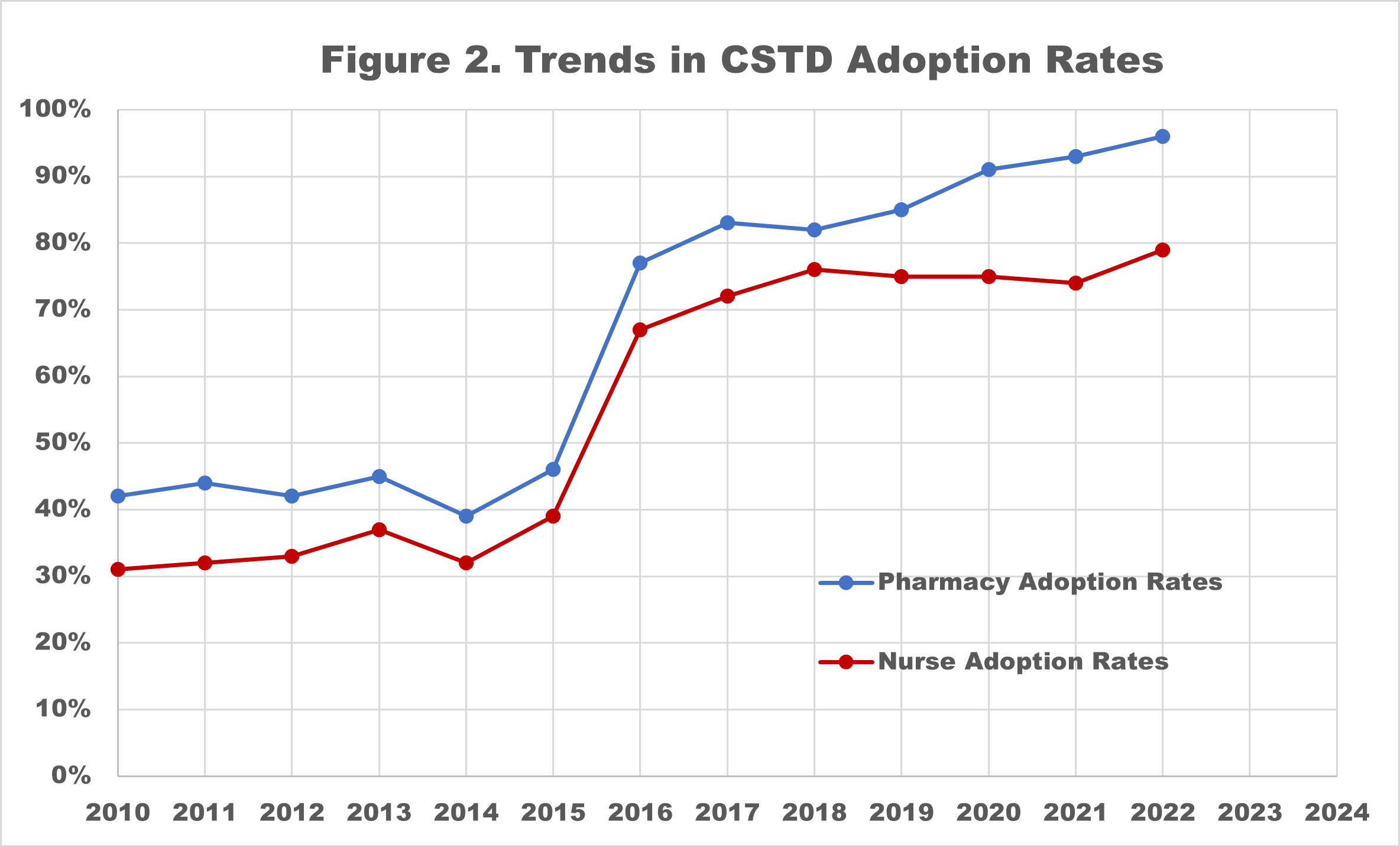
With the 2014 proposal and 2016 publishing of the original USP <800> Hazardous Drugs – Handling in Healthcare Settings, CSTD adoption rates jumped from 39% (2014) to 77% (2016) in the pharmacy and from 32% (2014) to 67% (2016) for nursing. We remained steadfast in our commitment to using CSTD devices in the pharmacy with adoption rates climbing to 96% in April 2022. Disappointingly, CSTD adoption rates for nursing improved only modestly, and declined in 2021 to 74%, before a small improvement to 79% in the 2022 survey. This data suggests a staggering 21% of nurses remain unprotected.
Hazardous Drug Safety & USP <800>
Advances in CSTD compatibility are enabling expanded protection for nursing and allowing pharmacy to adopt a passive hazardous drug safety program. The Arisure® CSTD has made protecting HCWs from HD exposure and ensuring compliance with USP <800> CSTD requirements simple. The Arisure® CSTD protects HCWs from HD exposure using novel needle-free, dual membrane, airtight and leakproof technology proven to prevent the escape of harmful vapor and aerosols into the environment, and remaining microbiologically closed for up to 7 days. Critically, the Arisure® CSTD Closed Male Luer (CML) is compatible with the ubiquitous *BD SmartSite™ and *Baxter™ ONE-LINK & CLEARLINK connectors, present on BD Alaris™, Baxter™, and Zyno™ infusion pump sets, and widely used across the U.S. Together with these compatible connectors, the Arisure® CML achieves a dry, closed, leakproof infusion without the need for the nurse to add a special proprietary CSTD connector on the patient.
In a facility using IV sets that contain these compatible valves, a pharmacy can dispense the HD with the Arisure® CML on the end of the set or syringe, then the nurse connects the Arisure® CML directly to the compatible connector already on the patient to administer the infusion. This expanded compatibility allows the USP <800> CSTD requirement to be achieved passively, with no added action or interruption to nursing workflow. The Arisure® system operates using intuitive and secure luer-lock connections providing easy integration into pharmacy and nursing practices. Reducing the number of components required to deliver a HD infusion may also improve cost, storage logistics, and simplify compliance for a healthcare facility. Protecting HCWs from HD exposure and complying with USP <800> CSTD requirements does not have to be overcomplicated or cost prohibitive. With the Arisure® CML, hazardous drug safety can be delivered passively without interrupting patient care. In facilities that do not use these infusion pumps or compatible needle-free connectors, the Arisure® Neutral Valve can be included in the HD safety program to enable a dry, closed, leakproof infusion.
It is a devastating irony that the healthcare clinicians that dedicate their lives to caring for patients are being diagnosed with cancer at alarming rates, experiencing increased rates of infertility and birth defects, and have documented genetic, cellular and organ damage resulting from HD exposure. Historically changes in the regulatory landscape precipitate exponential practice changes in healthcare. Together with advances in CSTD compatibility that enable passive hazardous drug safety, HCWs are offered hope of a future where delivering life-saving medications doesn’t mean risking your own life.
1. All data compiled from annual State of Pharmacy Compounding survey results published by Pharmacy Purchasing & Products2010-2022.
*SmartSite is a trademark of Becton, Dickinson and Company, its subsidiaries or affiliates. Yukon Medical Inc. is not affiliated with Becton, Dickinson and Company, *ONE-LINK AND CLEARLINK are trademarks of Baxter International Inc, its subsidiaries or affiliates. Yukon Medical Inc. is not affiliated with Baxter International Inc.

